Abstract
Background
The ongoing Coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is having an enormous impact on society worldwide and is especially posing a threat to health in vulnerable patients, such as patients with immune deficiencies. It is expected that patients who received Chimeric Antigen Receptor T-cell (CAR T-cell) therapy for hematologic malignancies are at risk for poor outcomes after COVID-19 due to their severely immunocompromised state caused by prior cumulative immunochemotherapy, on-target/off-tumor B-cell depletion, hypogammaglobulinemia and ongoing cytopenias. Current data are limited to small case series and case reports. This study describes the clinical characteristics and outcomes of CAR T-cell therapy recipients after developing COVID-19 in the largest cohort to date.
Methods
In response to the COVID-19 pandemic, the European Society for Blood and Marrow Transplantation (EBMT) developed a special COVID-19 report form to capture data from all patients with COVID-19 after treatment with CAR T-cell therapy for hematologic malignancies. Only PCR positive SARS-CoV-2 diagnosed patients before June 1 st, 2021 were included. The aim of this study was to describe the clinical course after COVID-19 diagnosis and evaluate overall survival. Overall survival probabilities were calculated using the Kaplan Meier method. Factors associated with mortality after COVID-19 diagnosis were examined using a Cox proportional hazard model.
Results
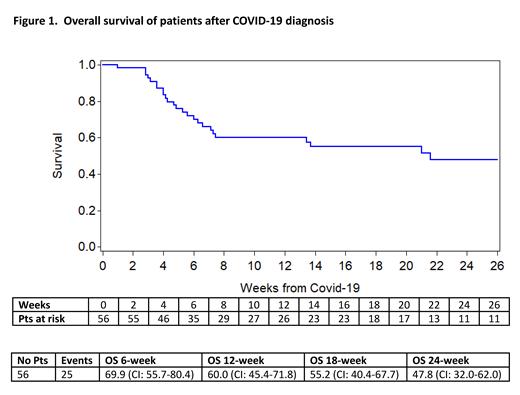
A total of 57 patients from 11 countries were reported to the EBMT. One patient with incomplete data at diagnosis and without any follow up information had to be excluded from the analysis. The median age of these 56 patients was 57.7 years (min-max 5.2 - 72.8) including 55 adults and one child. Of these patients, 32 were male. CAR T-cell therapy was given to 46 patients with B-cell-non-Hodgkin lymphoma, 7 patients with B-cell acute lymphoblastic leukemia, and 3 patients with multiple myeloma. The median time from CAR T-cell infusion to COVID-19 diagnosis was 7.4 months (min-max 0.03 - 25.3). At the time of COVID-19 diagnosis, 62.5% of patients were in complete remission, 12.5% of patients had a partial response and 25% of patients had relapsed/refractory disease. Forty-five patients (80%) were admitted to hospital (median 26,5 days, min-max 3-171) due to COVID-19. Of the admitted patients, 24 (53%) needed oxygen support. Twenty-two (49%) patients were admitted to the intensive care unit (median 14 days, min - max 2-65) and 16 (73%) of these patients received invasive ventilation. At the time of analysis, 25 of the 56 patients had died (44.6%), most (23/25) due to COVID-19, resulting in a COVID-19 attributable mortality rate of 41%. The Kaplan-Meier estimate of overall survival is shown in Figure 1. The median follow-up from COVID-19 diagnosis was 20.9 weeks. In 1 of the 32 alive patients there was no resolution of COVID-19 at the time of analysis. In multivariate analysis, older age (hazard ratio (HR) 1.50, 95% CI 1.11-2.03, p=0.009) and comorbidities (HR 2.56, 95% CI 1.05-6.23, p=0.001) had a negative impact on overall survival. Better performance status at time of admission (HR 0.72, 95% CI 0.59-0.88, p=0.038) had a positive impact on overall survival. Sex, time from CAR T-cell therapy to COVID-19 diagnosis, disease remission status and the occurrence of neurotoxicity or cytokine release syndrome after CAR T-cell infusion did not have a significant effect on overall survival in the multivariate analysis.
Conclusion
Patients with COVID-19 after B-cell-targeted CAR T-cell therapy have a very poor outcome. As it remains uncertain whether currently applied vaccination strategies against SARS-CoV-2 are effective after CAR T-cell therapy, vaccination of health-care personnel and family members in combination with protective measures against viral exposure are likely to play the most important role in protecting this vulnerable group of patients. Better treatment strategies are urgently needed.
Ljungman: OctaPharma: Other: DSMB; Enanta: Other: DSMB; Janssen: Other: Investigator; Takeda: Consultancy, Other: Endpoint committee, speaker; AiCuris: Consultancy; Merck: Other: Investigator, speaker. De La Camara: IQONE: Consultancy; Roche: Consultancy. Ortiz-Maldonado: Kite, Novartis, BMS, Janssen: Honoraria. Barba: Novartis: Honoraria; Gilead: Honoraria; BMS: Honoraria; Amgen: Honoraria; Pfizer: Honoraria. Kwon: Novartis, Celgene, Gilead, Pfizer: Consultancy, Honoraria. Sesques: Novartis: Honoraria; Chugai: Honoraria; Kite, a Gilead Company: Honoraria. Bachy: Kite, a Gilead Company: Honoraria; Novartis: Honoraria; Daiishi: Research Funding; Roche: Consultancy; Takeda: Consultancy; Incyte: Consultancy. Di Blasi: Kite, a Gilead Company: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Thieblemont: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses , Research Funding; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Hospira: Research Funding; Bayer: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses . Mutsaers: BMS: Consultancy; AstraZeneca: Research Funding. Nicholson: Kite, a Gilead Company: Other: Conference fees, Speakers Bureau; Novartis: Consultancy, Other: Conference fees; BMS/Celgene: Consultancy; Pfizer: Consultancy. Martínez-López: Janssen, BMS, Novartis, Incyte, Roche, GSK, Pfizer: Consultancy; Roche, Novartis, Incyte, Astellas, BMS: Research Funding. Ribera: NOVARTIS: Consultancy, Speakers Bureau; TAKEDA: Consultancy, Research Funding, Speakers Bureau; ARIAD: Consultancy, Research Funding, Speakers Bureau; SHIRE: Consultancy, Speakers Bureau; AMGEN: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau. Sanderson: Kite, a Gilead Company: Honoraria; Novartis: Honoraria. Bloor: Kite, a Gilead Company: Honoraria; Novartis: Honoraria. Ciceri: IRCCS Ospedale San Raffaele: Current Employment. Ayuk: Novartis: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Mallinckrodt/Therakos: Honoraria, Research Funding; Gilead: Honoraria; Miltenyi Biomedicine: Honoraria; Celgene/BMS: Honoraria. Kröger: Novartis: Research Funding; Riemser: Honoraria, Research Funding; Sanofi: Honoraria; Neovii: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Gilead/Kite: Honoraria; Celgene: Honoraria, Research Funding; AOP Pharma: Honoraria. Kersten: Celgene: Research Funding; Miltenyi Biotec: Consultancy, Honoraria, Other: Travel support; Roche: Consultancy, Honoraria, Other: Travel support, Research Funding; BMS/Celgene: Consultancy, Honoraria; Takeda: Research Funding; Novartis: Consultancy, Honoraria, Other: Travel support; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding. Mielke: DNA Prime SA: Speakers Bureau; Immunicum: Other: Data safety monitoring board; Novartis: Speakers Bureau; Miltenyi: Other: Data safety monitoring board; Gilead/KITE: Other: Travel support, Expert panel ; Celgene/BMS: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal